Karuna Therapeutics
Karuna Therapeutics (NASDAQ:KRTX) is a clinical-stage biotech headquartered in Boston, MA, founded back in July 2009 by Andrew Miller, Eric Elenko, and Peter Jeffrey Conn. The company focuses on the research and development of therapies that harness muscarinic cholinergic receptors, aiming to treat psychosis and cognitive impairment across a variety of central nervous system disorders.
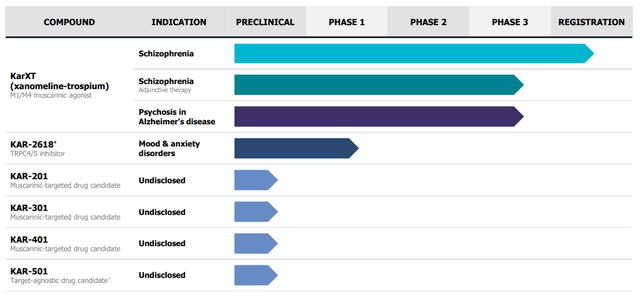
Company pipeline (Company IR deck)
Their lead asset, KarXT, has a unique muscarinic modulation approach that presents a differentiated clinical profile compared to first and second-generation antipsychotics that have been used for multiple decades. We see its lead candidate as a paradigm changer in the schizophrenia market based on a) the promising efficacy in the phase III studies supporting the therapeutic advantage (Efficacy and safety) and b) the long-term safety of xanomeline, highlighting KRTX’s potential.
Investment thesis
Our key investment thesis for Karuna Therapeutics hinges on several critical factors that underscore the potential of KarXT. Firstly, KarXT is in an advanced stage in its product development, with two successful schizophrenia studies (EMERGENT-1 and EMERGENT-2) substantiating our confidence in the efficacy and safety needed for approval. We believe the unique appeal of KarXT revolves around its novel/differentiated mechanism, distinct from traditional treatments, as it avoids blocking dopaminergic and serotonergic receptors. This innovation could usher in widespread effectiveness across the spectrum of schizophrenia symptoms, for example, positive, acute/secondary negative, and cognitive symptoms (negative and cognitive symptoms are not treatable with the current standard of care) – while also presenting a safety profile that avoids the typical side effects like weight gain, sedation, and movement disorders associated with current antipsychotic medications.
Furthermore, we see KarXT’s usage in treatment-resistant schizophrenia as a potential add-on therapy to currently available standard-of-care treatments. Of note, only clozapine is approved for such use, and its market ramp is limited due to undesirable side effects.
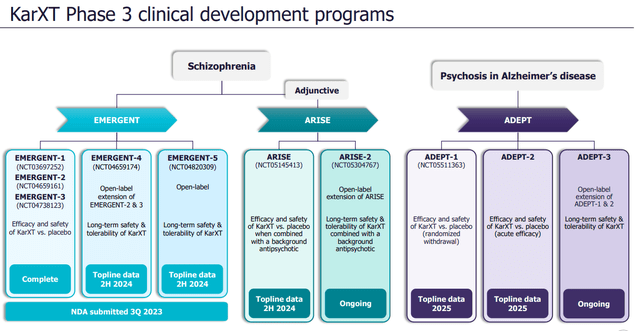
Phase 3 programs (Company publication)
Besides the schizophrenia franchise, we are a big fan of the potential for KarXT in Alzheimer’s disease psychosis (ADP), which offers diversification to the investment thesis.
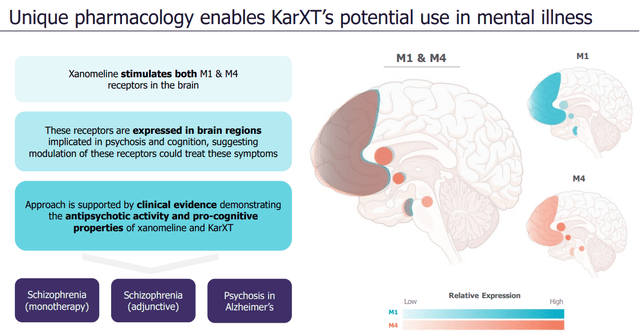
KarXT’s MOA (Company sources)
Schizophrenia background
The pathophysiology of schizophrenia is complex and not completely understood, but it is believed to involve a variety of genetic, environmental, neurobiological, and psychological factors. The illness is typically characterized by a disruption in the thought processes, perceptions, emotional responsiveness, and social interactions of the affected individual.
Pathophysiology of Schizophrenia:
Schizophrenia is associated with dysregulation of several neurotransmitter systems, including dopamine, glutamate, and serotonin. Here’s how the neurotransmitter systems are implicated:
-
Dopamine Hypothesis: This is one of the oldest hypotheses in the pathophysiology of schizophrenia. It suggests that schizophrenia results from an overactivity of dopamine neurotransmission in certain brain pathways. Specifically, excessive dopaminergic activity in the mesolimbic pathway is thought to be associated with positive symptoms of schizophrenia, such as hallucinations and delusions, while underactivity in the mesocortical pathway is linked to negative symptoms and cognitive deficits.
-
Glutamate Hypothesis: This suggests that hypofunction of the glutamate neurotransmitter system, particularly involving the N-methyl-D-aspartate (NMDA) receptor, may contribute to the symptoms of schizophrenia. Glutamate is the primary excitatory neurotransmitter in the brain, and its dysfunction could lead to a cascade of effects on other neurotransmitter systems.
-
Serotonin Hypothesis: Serotonin is another neurotransmitter involved in schizophrenia. Alterations in serotonergic transmission are thought to modulate the dopaminergic system and contribute to the symptoms of the disorder.
How current standard-of-care drugs work
Antipsychotic and Atypical Antipsychotics: Antipsychotic medications, also known as neuroleptics, are divided into two categories: typical (first-generation) and atypical (second-generation).
-
Typical Antipsychotics: These drugs primarily block D2 dopamine receptors, reducing the dopaminergic activity believed to underlie many of the positive symptoms of schizophrenia. However, because they are not selective and affect dopamine receptors throughout the brain, they can cause significant side effects, such as extrapyramidal symptoms (EPS), tardive dyskinesia, and increased prolactin levels.
-
Atypical Antipsychotics: These newer medications have a broader mechanism of action. They not only block D2 receptors but also affect serotonin receptors, particularly the 5-HT2A subtype. This serotonergic activity is thought to help alleviate negative and cognitive symptoms and to reduce the risk of EPS and tardive dyskinesia. However, atypical antipsychotics can lead to other side effects, such as weight gain, metabolic syndrome, and increased cardiovascular risk.
KarXT’s Mechanism of Action: KarXT (xanomeline-trospium) is designed to target the muscarinic cholinergic receptors, which represents a novel approach in the treatment of schizophrenia. Unlike typical or atypical antipsychotics, KarXT’s mechanism does not involve direct blockade of dopamine or serotonin receptors. Instead, it modulates the muscarinic receptors, which are involved in cognitive function and processing, as well as in modulating dopaminergic and glutamatergic systems indirectly.
The idea is that by targeting the muscarinic receptors (specifically M1/M4), KarXT can improve both positive and negative symptoms as well as cognitive deficits in schizophrenia without causing the dopaminergic-related side effects commonly seen with other antipsychotic drugs. This could represent a significant advancement in the treatment landscape of schizophrenia, potentially offering benefits for patients who are not adequately managed by current treatments or who suffer from their considerable side effects.
Key Unmet Needs:
- Reducing Side Effects: Both generations of antipsychotics come with significant side effects that can affect patient compliance and quality of life.
- Treatment for Negative and Cognitive Symptoms: Current treatments mainly address the positive symptoms of schizophrenia. There is a need for medications that also improve negative and cognitive symptoms, which KarXT aims to do.
- Safety and Tolerability: Enhanced safety and tolerability are critical, especially for the long-term management of schizophrenia.
KarXT’s unique MOA offers superior safety
Unlike the dopamine-centric mechanism of current antipsychotics, KarXT’s focus on muscarinic receptors provides a novel pathway to address symptoms of schizophrenia. This different mechanism has the potential to treat symptoms without many of the side effects associated with dopaminergic medications. Dopaminergic medications, especially those used to treat schizophrenia and other psychotic disorders, come with a range of adverse effects (AE). Here are some of the key adverse effects associated with dopaminergic antipsychotic medications:
-
Extrapyramidal Symptoms (EPS):
- Tardive Dyskinesia (TD): Involuntary, repetitive body movements that can be permanent. This is a significant concern with long-term use of typical (first-generation) antipsychotics.
- Parkinsonism: Symptoms resembling Parkinson’s disease, such as tremors, rigidity, and bradykinesia.
- Akathisia: A distressing restlessness that can lead to constant movement or an inability to sit still.
- Dystonia: Painful and prolonged muscle contractions.
-
Hyperprolactinemia: Elevated levels of prolactin in the blood, which can lead to symptoms such as breast enlargement, milk secretion, and menstrual irregularities in women and reduced sexual desire and erectile dysfunction in men.
-
Neuroleptic Malignant Syndrome (NMS): A rare but life-threatening reaction to antipsychotic drugs. Symptoms include hyperthermia, muscle rigidity, altered mental status, and autonomic dysfunction.
-
Metabolic Side Effects: Especially seen with atypical (second-generation) antipsychotics. These include weight gain, diabetes mellitus, and dyslipidemia.
-
Sedation: Many antipsychotics can cause drowsiness or sedation, which can affect daily activities.
-
Orthostatic Hypotension: A sudden drop in blood pressure when standing up, which can lead to dizziness or fainting.
-
Cardiac Effects: Some antipsychotics are associated with prolongation of the QT interval, which can lead to potentially life-threatening heart rhythm abnormalities.
As for KarXT, it operates primarily through muscarinic cholinergic receptors, providing a distinct mechanism of action compared to dopaminergic antipsychotics. In clinical trials, KarXT has demonstrated a favorable safety profile and has not shown many of the typical side effects associated with dopaminergic medications, especially extrapyramidal symptoms.
Efficacy: KarXT also targets negative symptoms
KarXT is indicated as a next-generation agent with a novel MoA targeting muscarinic receptors (M1/M4); the MoA of KarXT might offer better efficacy for negative and cognitive symptoms, which are currently underserved by existing medications (current agents only treat positive symptoms).
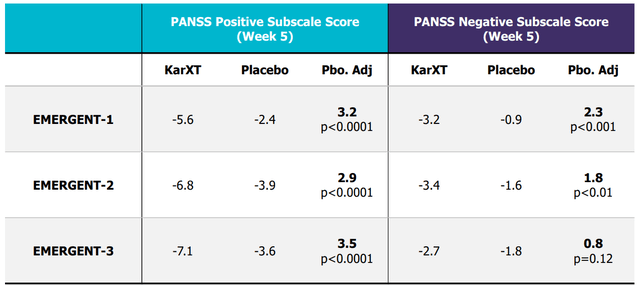
Trial data summary (Company source )
Many positive catalysts on the horizon
The Phase 1b trial assessing KarXT’s effect on 24-hour ambulatory blood pressure completed enrollment in 2Q23 with top-line data due in mid-4Q23.
Karuna Therapeutics completed the New Drug Application (NDA) submission for its lead product, KarXT, in late September.
The FDA decision on the NDA review is expected by the end of Nov, with a potential approval decision in 2H24, and we believe Karuna is gearing up for the potential U.S. launch of KarXT in 2H24. Based on the highly impressive EMERGENT trial results for treating psychosis associated with schizophrenia, we believe KarXT’s unique clinical profile is expected to drive its rapid adoption in the market (if approved), especially as an adjunctive therapy combined with existing approved agents.
Furthermore, we note that the company is awaiting top-line data from the EMERGENT-4, EMERGENT-5, and ARISE trials in 2H24. The ADEPT trials’ data targeting psychosis in Alzheimer’s patients will be released in 2025. In terms of the early stage development front, during Q3 2023 earnings, Karuna outlined its plans for another candidate, KAR-2618, targeting mood and anxiety disorders. A Phase 1b trial is planned for next year, focusing on major depressive disorder (MDD).
Financial Position: robust cash runway
The company reported a balance of approximately $1.3B in cash and short-term investments at the end of 3Q23. This funding is expected to last until the end of 2026, which is post the anticipated KarXT U.S. launch. In terms of OPEX, R&D expenditures for 3Q23 were approximately $104M, higher than the projected $94M. General and administrative spending was $32.3M against an estimated $30M. Importantly, considering that the annual cash burn is around $450M, we see ~2 years of cash runway for the company.
Valuation is attractive
The company’s current enterprise value is roughly US$5.7Bn; using the street consensus of $2.726Bn and an industry-standard peak sales multiple of x3, we get $8.1Bn implied enterprise value, which means there is roughly 30-40% upside to the current valuation.
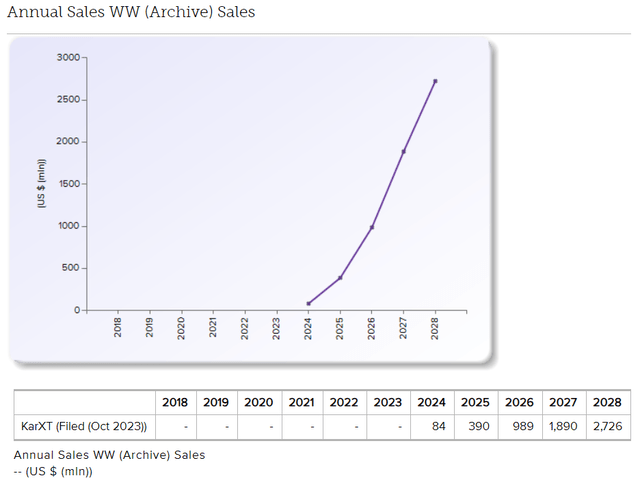
Broker consensus (Evaluate Pharma )
Risks
While Karuna’s pioneering approach to neuropsychiatric treatments holds great promise, they are not without challenges. The risks range from potential developmental and regulatory hurdles to financial constraints as a mid-cap entity without a commercial product (potential dilution risk through public offering). However, considering robust clinical data on hand, high unmet need, and a long cash runway with a strong cash position, we believe the risk is fairly de-risked and see Q4 2023 as an excellent time to establish a position for long-term investors considering the major sell-off we have seen post the pivotal trial readout.
Conclusion
We initiate Karuna with a buy rating considering a) novel mechanism of action and robust clinical data de-risking its approval, b) highly-attractive schizophrenia market opportunity, and c) attractive valuation with 30-40% upside after the recent sell-off and solid cash runway (~YE 2026). We are a buyer of the impressive/novel technology of Karuna therapeutics; while the standard of care for schizophrenia predominantly centers on modulating dopamine and, to some extent, serotonin pathways, KarXT introduces a fresh approach by targeting the muscarinic cholinergic system. This offers the potential for a differentiated efficacy and safety profile compared to existing treatments. Importantly, the company’s management team brings a wealth of expertise in neuropsychiatric drug development, especially around xanomeline, which further de-risks the investment thesis.
Read the full article here








Leave a Reply